Sustainable protein from Africa
The CSIR has developed a sustainable bioprocess to manufacture a fungal-based protein product for a South African business called MycoSure.
Dr Ghaneshree Moonsamy, a fungal expert at the CSIR’s Biomanufacturing Industry Development Centre (BIDC), says protein production in a bioreactor offers many sustainability and cost advantages over livestock and crop protein production.
She says MycoSure will be leveraging this technology using fungal protein, for the first time in Africa, to help address global food security.
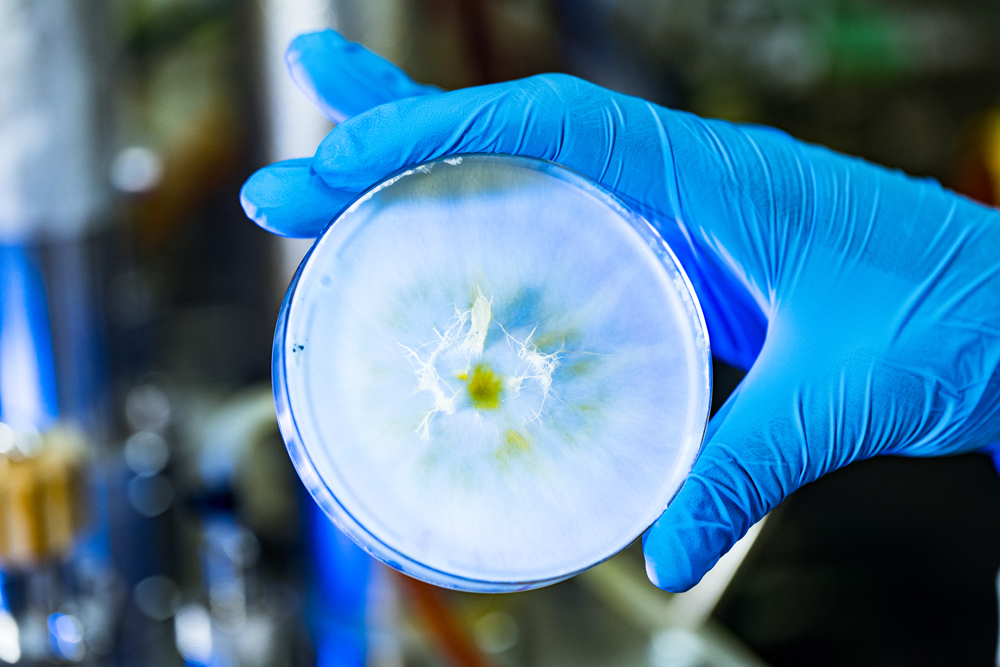
"The starting material for the protein product is Fusarium venenatum, a fungus known to have a high-protein content,” says Moonsamy.
This organism’s fruiting body is similar to other fungal fruiting bodies, including the common mushroom. A fruiting body usually sprouts from a mycelium, which is a network of fungal threads in soil or on a substrate. But when F. venenaturm is grown in a liquid culture, only mycelium threads grow.
The mycelium is the protein biomass, also known as mycoprotein, which can be dehydrated and packaged as a powdered food supplement. The CSIR recently completed this technology development for MycoSure and has licensed it to them.
Charles Reed, Mycosure’s CEO, says current global predictions put protein shortages at 250 million tonnes per year by 2025. For him, the question prior to partnering with the CSIR was how they could help solve that shortage “within planetary constraints”.
“When you look at the more conventional plant-based proteins like soy and maize, you need large areas of land and these plants have specific growth cycles that rely on consistent seasonal patterns and irrigation,” says Moonsamy. “There are so many other factors that could influence your yield in the field, whereas in a bioreactor where you're growing the fungi, you have a lot more control over your production process.”
The same goes for cultivated animal protein, with beef farming in particular contributing to excessive methane emissions that exacerbate climate change.
Moonsamy says the technology for fungal protein production addresses many of these environmental concerns and is already at a cost-competitive stage.
This is because scientists can reduce the cost of manufacturing by using raw materials that are locally available or that can be valorised from other industries, and because the process can be optimised to reduce water and energy consumption.
“The water used to clean the bioreactor, for example, can be recycled and reused up to three times, prior to discharge,” says Moonsamy.

"The starting material for the protein product is Fusarium venenatum, a fungus known to have a high-protein content,” says Moonsamy.
This organism’s fruiting body is similar to other fungal fruiting bodies, including the common mushroom. A fruiting body usually sprouts from a mycelium, which is a network of fungal threads in soil or on a substrate. But when F. venenaturm is grown in a liquid culture, only mycelium threads grow.
The mycelium is the protein biomass, also known as mycoprotein, which can be dehydrated and packaged as a powdered food supplement. The CSIR recently completed this technology development for MycoSure and has licensed it to them.
Charles Reed, Mycosure’s CEO, says current global predictions put protein shortages at 250 million tonnes per year by 2025. For him, the question prior to partnering with the CSIR was how they could help solve that shortage “within planetary constraints”.
“When you look at the more conventional plant-based proteins like soy and maize, you need large areas of land and these plants have specific growth cycles that rely on consistent seasonal patterns and irrigation,” says Moonsamy. “There are so many other factors that could influence your yield in the field, whereas in a bioreactor where you're growing the fungi, you have a lot more control over your production process.”

The same goes for cultivated animal protein, with beef farming in particular contributing to excessive methane emissions that exacerbate climate change.
Moonsamy says the technology for fungal protein production addresses many of these environmental concerns and is already at a cost-competitive stage.
This is because scientists can reduce the cost of manufacturing by using raw materials that are locally available or that can be valorised from other industries, and because the process can be optimised to reduce water and energy consumption.
“The water used to clean the bioreactor, for example, can be recycled and reused up to three times, prior to discharge,” says Moonsamy.


