Human and Societal Systems

ENHANCING HEALTHCARE SERVICES: THE IMPACT OF INNOVATIVE POINT-OF-CARE DEVICES
The human and societal systems research group focuses on point-of-care medical devices that empower health workers at primary healthcare clinics to conduct screening tests that were historically only possible at secondary and tertiary hospitals. For example, the UmbiflowTM system, which is used during the third trimester of pregnancy at around 30 weeks of gestational age, has seen drops in the still-birth rate by up to 50% in the communities where it was implemented.
Impacting the world's ante-natal stillbirth rate through medical ultrasound
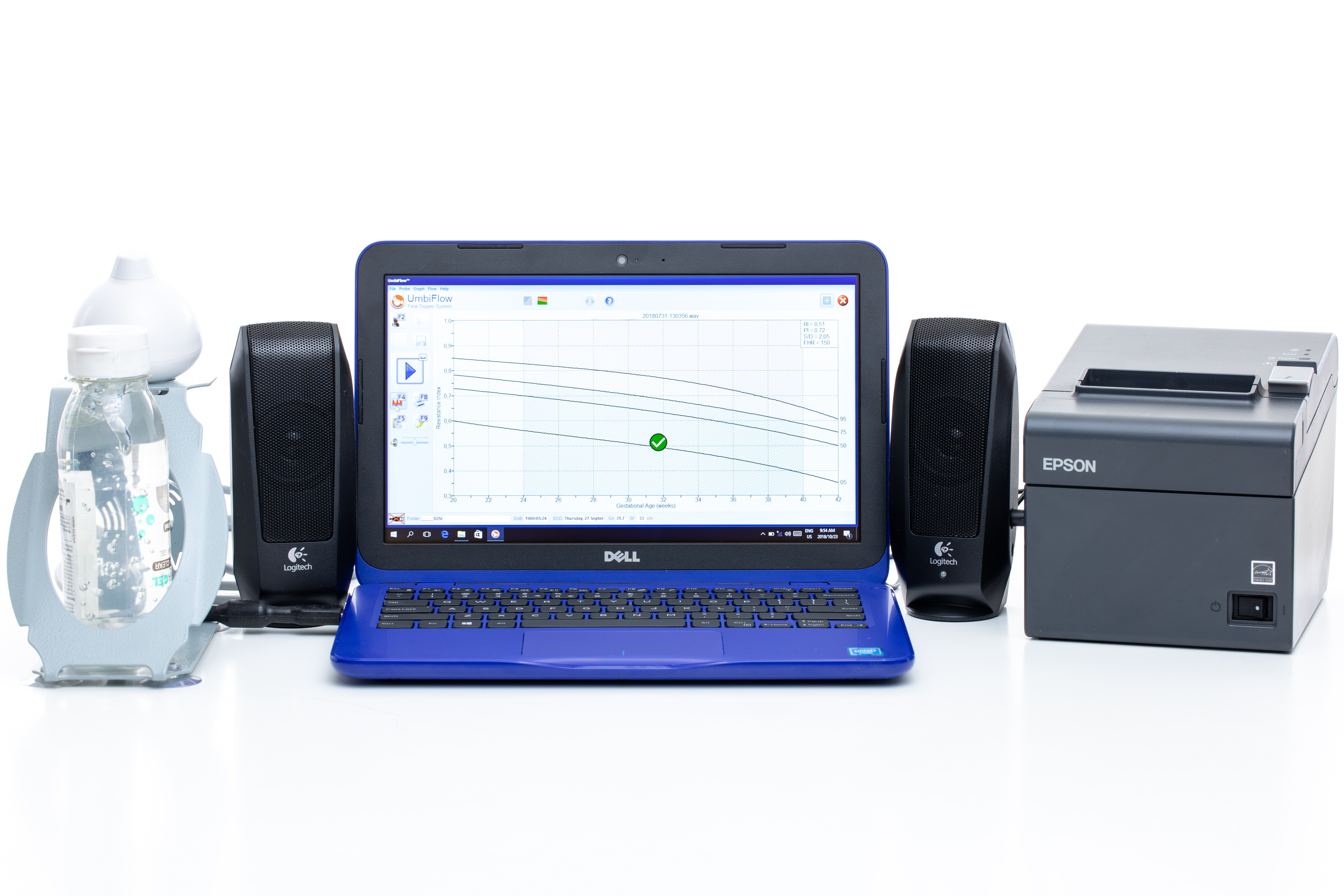
In collaboration with the South African Medical Research Council, a low-cost and easy-to-use Doppler ultrasound system was developed for use by registered nurses at primary health care clinics in low- and middle-income countries. The UmbiflowTM system measures the velocity of the blood flowing through the umbilical artery between a mother and her fetus and can provide a recommendation for referral in cases where a fetus is potentially at risk of being small for gestational age.
In a clinical study conducted in South Africa’s Tshwane District on 2 800 third-trimester pregnant mothers, the stillbirth rate was decreased by approximately 50% through the use of technology, which is a significant clinical result.
UmbiflowTM is currently being lined up for clinical tests in a range of low- and middle-income countries to check prevalence and impact, and it is presently being tested in primary health care clinics in all nine of South Africa’s provinces.
The CSIR’s skills are in the development of the high-frequency ultrasound probe, analogue and digital electronic components used to interface the transducer to a computer via a USB port, user interface software development, signal processing aspects and remote communication of the data to a central server. The CSIR conducts its medical device development work under an ISO 13485 medical quality system that lends itself to CE and FDA approvals.

