What we do
Contact information:
Highlights

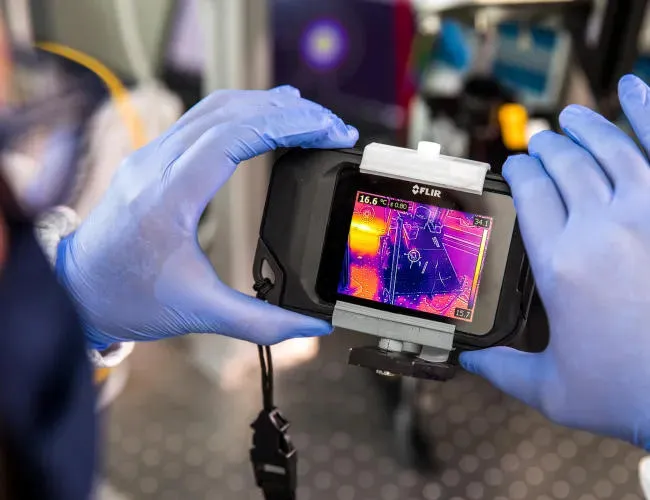
Production of further diamond sorting lasers for De Beers Ignite
Five bespoke laser systems have been integrated into diamond sorting systems at De Beers Ignite mines in Botswana. To the naked eye, diamonds and quartz appear similar. However, Raman spectroscopy can detect molecular structures, making it possible to distinguish between the two despite their similar molecular signatures. The CSIR system utilises Raman spectroscopy to accurately differentiate diamonds from quartz.

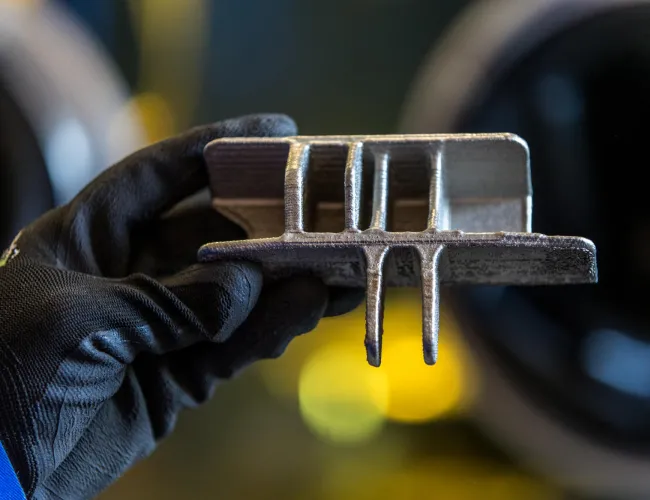
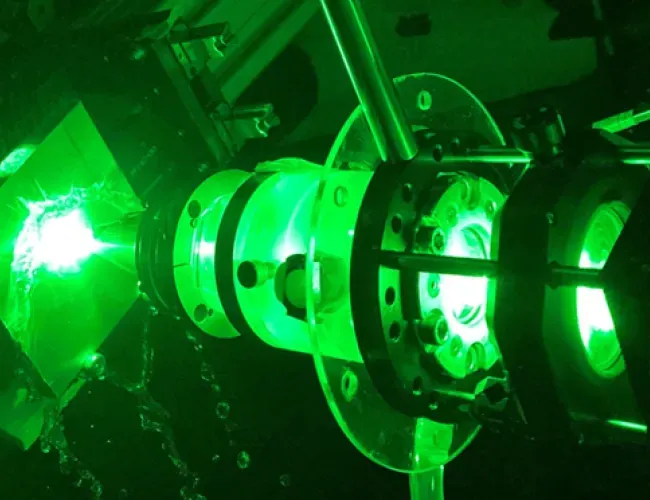
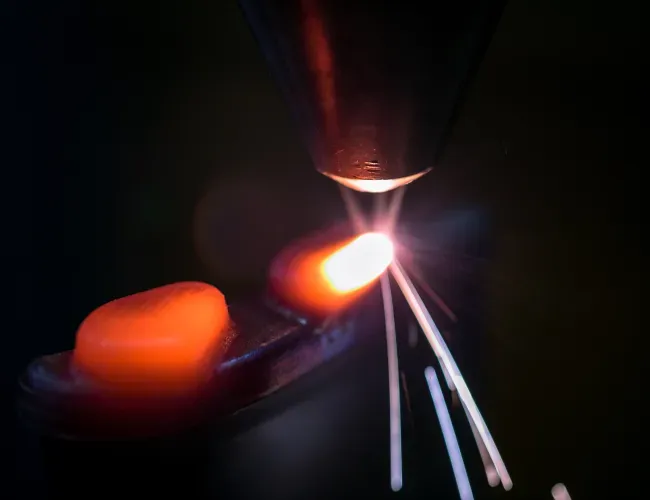
Laser-beam welding technology for repairs to Eskom power generation equipment
The CSIR leveraged its laser beam welding capabilities to perform repairs at several Eskom power stations. The technology was developed in collaboration with Eskom’s Research, Testing and Development department. The refurbishment process used laser cladding, a technique in which metal is deposited and welded precisely onto worn components. Lasers are particularly well suited to this method due to their low heat input, high speed and the precision enabled by a robotically controlled arm.
Our research
Our facilities
National programmes
We host collaborative initiatives involving research organisations, higher education institutions and stakeholders from the public and private sectors. These partnerships focus on research, development and the application of technologies to advance the photonics industry and strengthen the skills base.